FastFit Anchor Razek – Adjustable EN
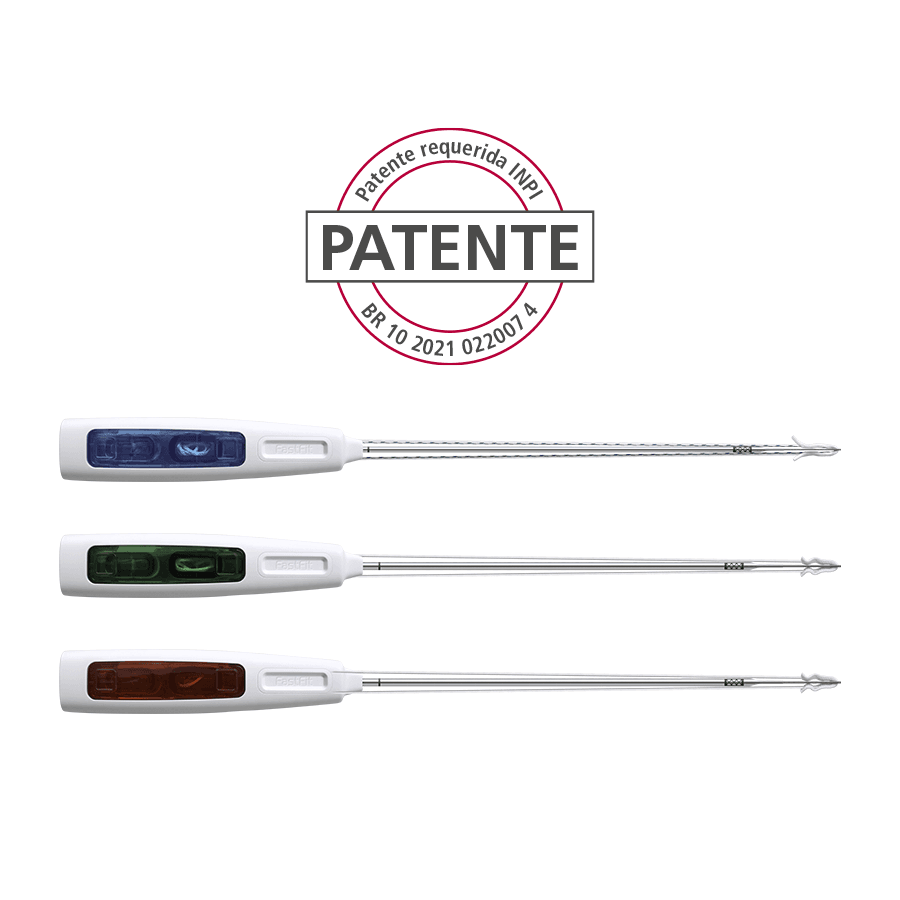
Products Home Products ANVISA No.: 80356130205Images for illustrative purposes only FastFit Anchor Razek – Adjustable The FastFit Anchor Razek flexible suture anchor family is indicated for use in arthroscopic or conventional orthopaedic surgical procedures on the upper and lower limbs, in which sutures are used to join soft tissues such as ligaments, tendons or joint capsules to the bone. Its design is based on the principles of minimal aggression and greater holding power. Reduced bone removal – the smaller diameter drill makes the hole created for the implant much smaller. As a result, there is greater preservation of the bone-tissue contact surface, as well as less aggression, considerably reducing post-surgical pain; Complications such as fractures, ostolysis and synovitis, which can occur with other materials, especially bioabsorbable materials, are minimized. The reduced diameter of the anchor reduces the need for larger bone perforations, which are common with metal, peek and bioabsorbable anchors, minimizing complications such as bone fragility and relesions. In addition, in cases where more anchors are needed, there will be less chance of complications and a greater possibility of their proper positioning; With the implant based entirely on sutures, the possibility of intra-articular free bodies that can occur in other types of implants is eliminated. ANVISA No.: 80356130205Images for illustrative purposes only Technical specifications • Single-use product • Sterilization validity: 3 years • Available in three diameters: 1.6 mm, 2.0 mm and 2.5 mm – loaded with very high-strength wires (UHMWPE * ForceFiber® Teleflex*); Models FASTFIT ANCHOR RAZEK – 1.6 mm Adjustable1.6 Adjustable (FAA 11-16S) – (Code 500120050)1.6 Adjustable (FAA 11-16M) – (Code: 500120055)1.6 Adjustable (FAA 11-16L) – (Code 500120057) FASTFIT ANCHOR RAZEK – 2.0 mm Adjustable2.0 Adjustable (FAA 12-20M) – (Code 500120040)2.0 Adjustable (FAA 12-20L) – (Code 500120045) FASTFIT ANCHOR RAZEK – 2.5 mm Adjustable2.5 Adjustable (FAA 12 25M) – (Code 500120100)2.5 Adjustable (FAA 12 25L) – (Code 500120105) MANUAL CATALOG VIDEO DOUBT Others Products edit post edit post edit post edit post
Percutaneous Lumbar Discectomy and E-Access Kit

Products Home Products ANVISA No.: 80356130109Images for illustrative purposes only The Percutaneous Lumbar Discectomy and E-Access Kit, together with a shaver, has the function of providing instruments for decompressing herniated intervertebral discs using a percutaneous method (Percutaneous Discectomy). It also enables endoscopic access using its Guide Cannula and Cutting Cannula, allowing the fibrous annulus to be ruptured using its Trephine. ANVISA No.: 80356130109Images for illustrative purposes only Technical specifications • Single-use product • Sterilization validity: 2 years Items Percutaneous Lumbar Discectomy and E-Access Kit(Code 921110000) • Trocar C• Obturator C• Trephine C• Guide Cannula• Nitinol Guide Wire• Drilling Irrigation Device• Bone Debridement Cannula RBC – 2.4 R MANUAL CATALOG VIDEO DOUBT Others Products edit post edit post edit post edit post
Razek Endoscopes for Arthroscopy

Products Home Products ANVISA No.: 80356130121Images for illustrative purposes only Razek Endoscopes for Arthroscopy are medical devices designed to visualize openings and cavities during arthroscopic surgery. These instruments should be used in conjunction with Duet HD, Triplet HD equipment or any other surgical equipment that requires clear images for the surgical procedure. Razek Sterilizing Case is not part of the product and can be purchased separately. ANVISA No.: 80356130121Images for illustrative purposes only Models Endoscope Angled endoscope The full list of models is presented in the user manual. MANUAL CATALOG VIDEO DOUBT Others Products edit post edit post edit post edit post
FastFit Anchor Razek (Model with tape)

Products Home Products ANVISA No.: 80356130205Images for illustrative purposes only FastFit Anchor Razek (Modelo com fita) The FastFit Anchor Razek flexible suture anchor family is indicated for use in arthroscopic or conventional orthopaedic surgical procedures on the upper and lower limbs, in which sutures are used to join soft tissues such as ligaments, tendons or joint capsules to the bone. Its design is based on the principles of minimal aggression and greater holding power. ANVISA No.: 80356130205Images for illustrative purposes only More Details Tape models offer a larger contact surface, providing superior clinical results*. The tapes are extremely resistant, made from ultra-high molecular weight polyethylene. *Maia Dias, C., Gonçalves, S.B., Completo, A. et al. Why are tapes better than wires in knotless rotator cuff repairs? An evaluation of force, pressure and contact area in a tendon bone unit mechanical model. J EXP ORTOP 8, 9 (2021). https://doi.org/10.1186/s40634-020-00321-y Technical specifications Single-use product Sterilization validity: 3 years Ø 1.6mm1.6 (FTB 12-16M) – (Code 500120260)1.6 (FTA 12-16M) – (Code 500120265)1.6 (FTB 12-16L) – (Code 500120280)1.6 (FTA 12-16L) – (Code 500120285) Ø 2.5mm2.5 (FTP 22-25M) – (Code 500120270)2.5 (FTP 22-25L) – (Code 500120290) Model with Needle2,5 (FTP 22-25MA) – (Code 500120275) Inserting device shank lengthM: 145 mm / L: 255 mmTape Width: 1.5 mm MANUAL CATALOG VIDEO DOUBT Others Products edit post edit post edit post edit post
Inside Vision – EN

Products Home Products Inside Vision ANVISA No.: 80356139023Images for illustrative purposes only Inside Vision Inside Vision is indicated for access to the spine in endoscopic spinal surgery (endoscopic spinal surgery), spinal decompression and/or cauda equina and in surgical biopsy of the spine. By using it, the surgeon has a series of support devices that allow access, visualization and the passage of instruments needed to perform endoscopic spinal surgical procedures in a practical and safe way. ANVISA No.: 80356139023Images for illustrative purposes only Technical specifications • Single-use product • Sterilization validity: 2 years Items Inside Vision(Code 921280000) • Straight Access Sleeve• 30° Access Sleeve • 70° Access Sleeve• Conical Dilator 1 Hole• Puncture Cannula• Nitinol Wire Guide MANUAL CATALOG VIDEO DOUBT Others Products edit post edit post edit post edit post
Serratus Long – EN
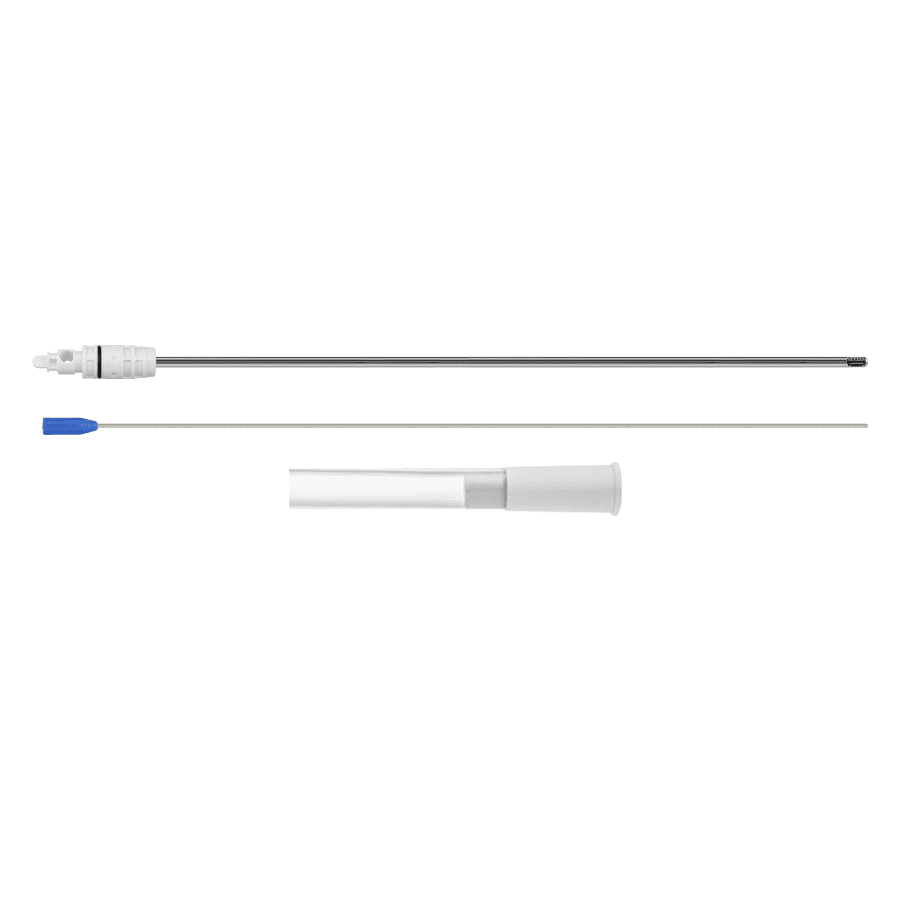
Products Home Products Urologia Serratus Long ANVISA No.: 80356139026Images for illustrative purposes only Serratus Long Serratus Long provides a method for performing prostate morcellation in urological endoscopic prostate enucleation procedures, and is indicated for the surgical treatment of benign prostatic hyperplasia via the urethral access route. The MPU Morcellation Cannula together with the Aspiration Tube, present in the Serratus Long, make it possible to perform automated morcellation after enucleation via the urethral access route without the need for incisions, thus reducing the patient’s recovery time. ANVISA No.: 80356139026Images for illustrative purposes only Technical specifications Single-use product Sterilization validity: 2 years Items Serratus Long(Code: 930400000) Morcellation Cannula MPUFitting type: RUseful size: Ø 4.5 x 390 mm Suction TubeSize: Øo 12 x Øi 8 mm x 3000 mm Accessory: Tissue remover MANUAL CATALOG VIDEO DOUBT Others Products edit post edit post edit post edit post
Spine EasyFlow – EN

Products Home Products Spine Easy Flow ANVISA No.: 80356130212Images for illustrative purposes only Spine EasyFlow Spine EasyFlow, has the function of providing instruments for decompression of intervertebral disc herniation and/or treatment of disc protrusions with partial rupture of annular fibers through an endoscopic and/or minimally invasive method (percutaneous discectomy). The product has instruments for performing automated nucleotomy, nucleoplasty, annulotomy and annuloplasty, as well as a complete set for accessing the disc nucleus. The Bipolar Resector Cannula present in the Spine EasyFlow has an irrigation line for serum, which guarantees cooling of the active tip and circulation of fluids, minimizing possible thermal damage to adjacent tissues. The puncture cannula in the Spine EasyFlow has an echogenic active tip (sonovisible), which allows it to be guided by ultrasound – ultrasonic guidance increases the accuracy and safety of the procedure and minimizes the patient’s exposure to fluoroscopy. ANVISA No.: 80356130212Images for illustrative purposes only Technical specifications • Single-use product • Sterilization validity: 2 years Items Inside Vision(Code 921280000) • Trocar• Obturator• Trephine• Guide Wire• Sonovisible Puncture Cannula• Bipolar Resecting Cannula with Cooling Model Spine EasyFlow L (Code 921310100) MANUAL CATALOG VIDEO DOUBT Others Product edit post edit post edit post edit post
FastFit Anchor Razek (models 1.5 and 1.5 Mini)
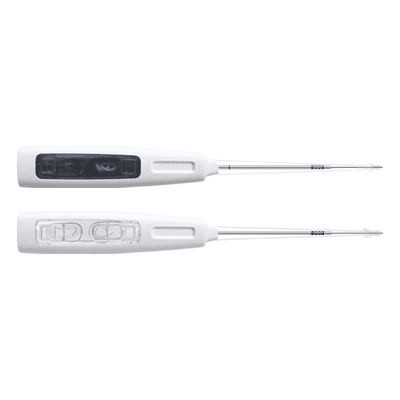
Products Home Products ANVISA No.: 80356130205Images for illustrative purposes only Indications for use: Temporomandibular Joint (TMJ), Foot and Ankle, Hand and Wrist FastFit Anchor Razek is a flexible suture anchor that guarantees maximum fixation with minimal bone aggression. The design is minimally invasive and uses highly resistant materials. This guarantees high stability and fewer complications in the post-operative period. The product’s instruments are purchased separately. ANVISA No.: 80356130205Images for illustrative purposes only Technical specifications • Single-use product • Sterilization validity: 3 years • Safe for use in an MRI environment Models • 1.5 Mini (FFM 10-15S) – (Code 500120115)• 1.5 (FFA 11-15S) – (Code 500120125) Adjustable models• 1.5 Mini Adjustable (FAM 10-15S) – (Code 500120110)• 1.5 Adjustable (FAA 10-15S) – (Code – 500120120) Models with needles• 1.5 Mini (FFM 10-15SA) – (Code 500120210)• 1.5 (FFA 11-15SA) – (Code 500120215) MANUAL CATALOG VIDEO DOUBT Others Products edit post edit post edit post edit post Kit Complementar para Cirurgia Endoscópica Interlaminar de Coluna Razek Kit de Instrumental para Parafuso de Compressão Canulado Razek Kit Complementar para Cirurgia Endoscópica de Coluna Razek L Kit para Correção de Desvios Ortopédicos Foot RZ System
SpineCare Endoscopy – EN

Products Home Products SpineCare Endoscopy ANVISA No.: 80356130213Images for illustrative purposes only SpineCare Endoscopy SpineCare Endoscopy has the function of providing an endoscopic method for spinal surgeries, in intervertebral disc herniation decompression procedures and/or treatment of disc protrusions with partial rupture of the annular fibers. The product has a complete set of instruments for performing automated nucleotomy, nucleoplasty, annulotomy and annuloplasty. The Bipolar Resector Cannula present in the SpineCare Endoscopy has an irrigation line for serum, which guarantees cooling of the active tip and circulation of fluids, minimizing possible thermal damage to adjacent tissues. The puncture cannula in the SpineCare Endoscopy has an echogenic active tip (sonovisible), which allows it to be guided by ultrasound – ultrasonic guidance increases the accuracy and safety of the procedure and minimizes the patient’s exposure to fluoroscopy. Using the Access Gloves in the SpineCare Endoscopy the surgeon has a series of translucent and radiotransparent support devices that allow access, visualization and passage of the instruments needed to perform endoscopic spinal surgical procedures, in a practical and safe manner. The Access Gloves in the SpineCare Endoscopy are made from translucent materials that give the surgeon full visualization of adjacent structures during access, increasing the safety of the procedure. In addition, the Access Gloves have a radiopaque stripe along their length, allowing their position to be visualized using fluoroscopy, and they also have an atraumatic geometry, designed in such a way as to minimize any kind of damage to adjacent soft tissues. ANVISA No.: 80356130213Images for illustrative purposes only Technical specifications • Single-use product • Sterilization validity: 2 years Items • Bipolar Resecting Cannula with Cooling• Irrigation Device• Sonovisible Puncture Cannula• Nitinol Wire• Bone Debridement Cannula• Straight Access Sleeve• Access Sleeve 30º• Access Sleeve 70º• Conical Dilator 1 Hole Models The full list of models is available in the user manual. MANUAL CATALOG VIDEO DÚVIDAS Others Products edit post edit post edit post edit post
PB DiscSys – EN

Products Home Products PB DiscSys ANVISA No.: 80356130199Images for illustrative purposes only PB DiscSys PB DiscSys provides a method for treating cervical, thoracolumbar and lumbar intradiscal decompression, performing coagulation, dissection or electrosurgical fulguration, while simultaneously promoting continuous fluid irrigation through the Trocar. Irrigation is carried out by gravity, but the flow can be amplified using the exclusive Flow Intensifier, as well as easily controlled by the surgeon using the Tweezer to interrupt the flow. Its polished, noble material active tip prevents tissue from sticking to it and transfers heat away from the tip, preventing adjacent tissues from overheating. These characteristics allow the structures to be preserved, avoiding possible bleeding and improving the post-operative period. ANVISA No.: 80356130199Images for illustrative purposes only Technical specifications • Sterile, single-use kit for the intradiscal access and decompression procedure; • Trocar with integrated irrigation controlled by the surgeon, which enables irrigation, insertion and removal of instruments; • It provides high precision in the performance of its functions, minimizing the chances of damage to local tissues; • Enables effective vaporization and coagulation with minimal blood loss; • Bipolar hemostasis forceps with an active tip that allows tissue to be clamped and an ergonomic handle that reproduces the natural clamping movement of the surgeon’s hand. Modeles • PB DiscSys – C (Code 881490200) – 300 mmHemostasis Bipolar 300 (1 piece);Trocar C (1 piece);Obturator C (1 piece);Trephine C (1 piece);Puncture Cannula (1 piece);Nitinol Guidewire (1 piece);Flow booster – Accessory (2 pieces). • PB DiscSys – L (Code 881490100) – 450 mmHemostasis Bipolar 450 (1 piece);Trocar L (1 piece);Obturator L (1 piece);Trephine L (1 piece);Puncture Cannula (1 piece);Nitinol Guide Wire (1 piece);Flow booster – Accessory (2 piece). MANUAL CATALOG VIDEO DOUBT Others Products edit post edit post edit post edit post

