Serratus Long
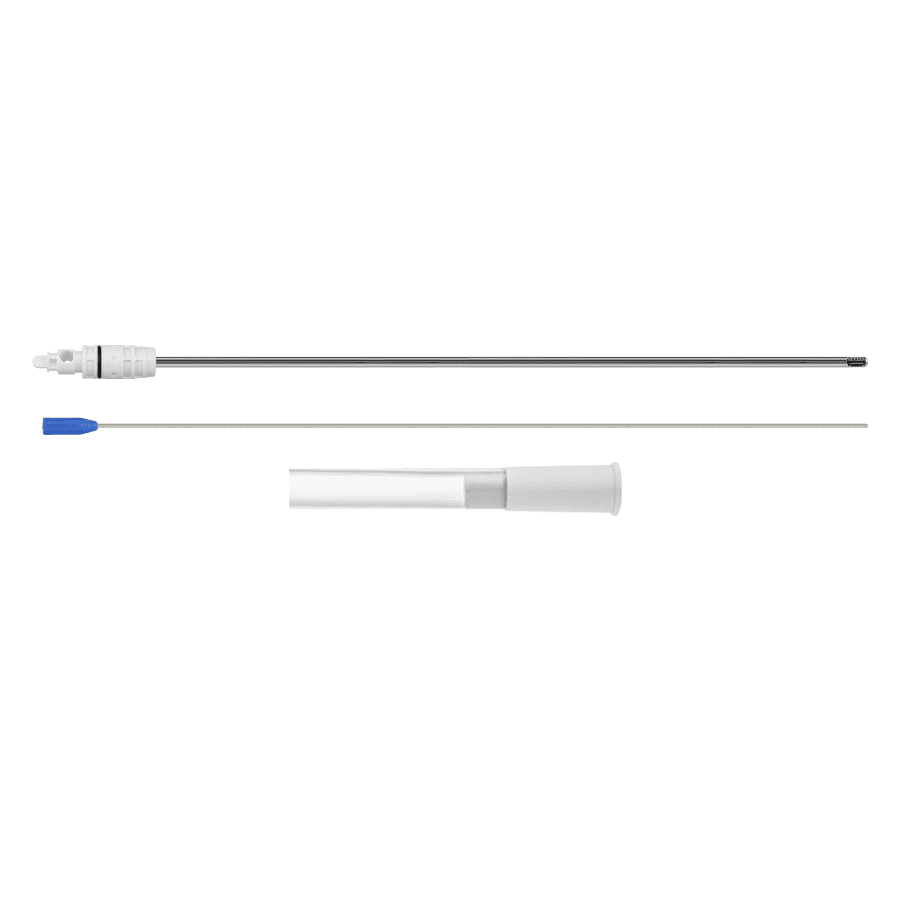
Home Products Urology Serratus Long ANVISA No.: 80356139026Images for illustrative purposes only Serratus Long Serratus Long provides a method for performing prostate morcellation in urological endoscopic prostate enucleation procedures, and is indicated for the surgical treatment of benign prostatic hyperplasia via the urethral access route. The MPU Morcellation Cannula together with the Aspiration Tube, present in the Serratus Long, make it possible to perform automated morcellation after enucleation via the urethral access route without the need for incisions, thus reducing the patient’s recovery time. ANVISA No.: 80356139026Images for illustrative purposes only Technical specifications Single-use product Sterilization validity: 2 years Items Serratus Long(Code: 930400000) • Morcellation Cannula MPUFitting type: RUseful size: Ø 4.5 x 390 mm • Suction TubeSize: Øo 12 x Øi 8 mm x 3000 mm Accessory: Tissue remover MANUAL CATALOG DOUBT VIDEO Related Products edit post Serratus Supra
Spine EasyFlow

Home Products Spine Easy Flow ANVISA No.: 80356130212Images for illustrative purposes only Spine EasyFlow Spine EasyFlow, has the function of providing instruments for decompression of intervertebral disc herniation and/or treatment of disc protrusions with partial rupture of annular fibers through an endoscopic and/or minimally invasive method (percutaneous discectomy). The product has instruments for performing automated nucleotomy, nucleoplasty, annulotomy and annuloplasty, as well as a complete set for accessing the disc nucleus. The Bipolar Resector Cannula present in the Spine EasyFlow has an irrigation line for serum, which guarantees cooling of the active tip and circulation of fluids, minimizing possible thermal damage to adjacent tissues. The puncture cannula in the Spine EasyFlow has an echogenic active tip (sonovisible), which allows it to be guided by ultrasound – ultrasonic guidance increases the accuracy and safety of the procedure and minimizes the patient’s exposure to fluoroscopy. ANVISA No.: 80356130212Images for illustrative purposes only Technical specifications • Single-use product • Sterilization validity: 2 years Items Spine EasyFlow(Code 921310100)• Trocar• Obturator• Trephine• Guide Wire• Sonovisible Puncture Cannula• Bipolar Resecting Cannula with Cooling MANUAL CATALOG DOUBT VIDEO Related Products edit post Razek Surgical Cutters edit post PB SafeBlend edit post Clear Acess edit post Razek Craniotomy Kit
SpineCare Endoscopy

Home Products SpineCare Endoscopy ANVISA No.: 80356130213Images for illustrative purposes only SpineCare Endoscopy SpineCare Endoscopy has the function of providing an endoscopic method for spinal surgeries, in intervertebral disc herniation decompression procedures and/or treatment of disc protrusions with partial rupture of the annular fibers. The product has a complete set of instruments for performing automated nucleotomy, nucleoplasty, annulotomy and annuloplasty. The Bipolar Resector Cannula present in the SpineCare Endoscopy has an irrigation line for serum, which guarantees cooling of the active tip and circulation of fluids, minimizing possible thermal damage to adjacent tissues. The puncture cannula in the SpineCare Endoscopy has an echogenic active tip (sonovisible), which allows it to be guided by ultrasound – ultrasonic guidance increases the accuracy and safety of the procedure and minimizes the patient’s exposure to fluoroscopy. Using the Access Gloves in the SpineCare Endoscopy the surgeon has a series of translucent and radiotransparent support devices that allow access, visualization and passage of the instruments needed to perform endoscopic spinal surgical procedures, in a practical and safe manner. The Access Gloves in the SpineCare Endoscopy are made from translucent materials that give the surgeon full visualization of adjacent structures during access, increasing the safety of the procedure. In addition, the Access Gloves have a radiopaque stripe along their length, allowing their position to be visualized using fluoroscopy, and they also have an atraumatic geometry, designed in such a way as to minimize any kind of damage to adjacent soft tissues. ANVISA No.: 80356130213Images for illustrative purposes only Technical specifications • Single-use product • Sterilization validity: 2 years Items • Bipolar Resecting Cannula with Cooling• Irrigation Device• Sonovisible Puncture Cannula• Nitinol Wire• Bone Debridement Cannula• Straight Access Sleeve• Access Sleeve 30º• Access Sleeve 70º• Conical Dilator 1 Hole Models The full list of models is available in the user manual. MANUAL CATALOG DÚVIDAS VIDEO Related Products edit post PB DiscSys edit post Razek Surgical Cutters edit post PB Hemo2Way edit post Maxcut
PB DiscSys

Home Products PB DiscSys ANVISA No.: 80356130199Images for illustrative purposes only PB DiscSys PB DiscSys provides a method for treating cervical, thoracolumbar and lumbar intradiscal decompression, performing coagulation, dissection or electrosurgical fulguration, while simultaneously promoting continuous fluid irrigation through the Trocar. Irrigation is carried out by gravity, but the flow can be amplified using the exclusive Flow Intensifier, as well as easily controlled by the surgeon using the Tweezer to interrupt the flow. Its polished, noble material active tip prevents tissue from sticking to it and transfers heat away from the tip, preventing adjacent tissues from overheating. These characteristics allow the structures to be preserved, avoiding possible bleeding and improving the post-operative period. ANVISA No.: 80356130199Images for illustrative purposes only Technical specifications • Sterile, single-use kit for the intradiscal access and decompression procedure; • Trocar with integrated irrigation controlled by the surgeon, which enables irrigation, insertion and removal of instruments; • It provides high precision in the performance of its functions, minimizing the chances of damage to local tissues; • Enables effective vaporization and coagulation with minimal blood loss; • Bipolar hemostasis forceps with an active tip that allows tissue to be clamped and an ergonomic handle that reproduces the natural clamping movement of the surgeon’s hand. Models • PB DiscSys – C (Code 881490200) – 300 mmHemostasis Bipolar 300 (1 piece);Trocar C (1 piece);Obturator C (1 piece);Trephine C (1 piece);Puncture Cannula (1 piece);Nitinol Guidewire (1 piece);Flow booster – Accessory (2 pieces). • PB DiscSys – L (Code 881490100) – 450 mmHemostasis Bipolar 450 (1 piece);Trocar L (1 piece);Obturator L (1 piece);Trephine L (1 piece);Puncture Cannula (1 piece);Nitinol Guide Wire (1 piece);Flow booster – Accessory (2 pieces). MANUAL CATALOG DOUBT VIDEO Related Products edit post Razek Surgical Cutters edit post Percutaneous Discectomy Cannula Kit L edit post US Irrigation and Suction Extension Device edit post Spine EasyFlow
Nano Guillotine

Home Products Nano Guillotine ANVISA No.: 80356139024Images for illustrative purposes only Nano Guillotine Nano Guillotine is a cannulated device for cutting, removing and suctioning tissues such as cartilage, fibrocartilage, synovial membranes and soft tissues in general, in different cavities and joints such as the shoulder, wrist, knee and ankle. The integrated storage chamber allows the removed tissue to be stored for later analysis by biopsy, quantification or visual analysis. Designed for use in space-saving surgeries that require tissue removal. ANVISA No.: 80356139024Images for illustrative purposes only Technical specifications • Single-use product • Sterilization validity: 2 years Models Nano Guillotine Kerr C – Size 80 mm x 3.5 mm (Code 921290000) Nano Guillotine Kerr L – Size 150 mm x 3.5 mm (Code 921290100) Nano Guillotine Wedge C – Size 80 mm x 3.5 mm (Code 921290200) Nano Guillotine Wedge L – Size 150 mm x 3.5 mm (Code 921290300) Nano Guillotine Hook C – Size 80 mm x 3.5 mm (Code 921290400) Nano Guillotine Hook L – Size 150 mm x 3.5 mm (Code 921290500) MANUAL CATALOG DOUBT VIDEO Related Products edit post Suture Needle edit post Puncture Cannula edit post Razek Surgical Cutters edit post Suture Fix Access Cannula
Razek Dissector tip – ATM

Home Products ANVISA No.: 80356130116Images for illustrative purposes only Razek Dissector Tip is used in monopolar surgical procedures. It is indicated for tissue ablation, coagulation and cutting. Razek Dissector Tip does not require an electrosurgical pen, as this is part of the product. ANVISA No.: 80356130116Images for illustrative purposes only Technical specifications • Single-use product • Sterilization validity: 3 years Model ATM 85 x 1.5 mm(Code 881443500) MANUAL DOUBT CATALOG VIDEO Related Products edit post Pain Cannula STP Plus Kit edit post HPF Diamond Drill edit post Razek Flush Tip edit post HPF Surgical Cutter
Razek Micro Saw Blades

Home Products ANVISA No.: 80356130059Images for illustrative purposes only Razek Micro Saw Blades have the function of making bone cuts in surgical procedures, in conjunction with the Power Micro Saws. ANVISA No.: 80356130059Images for illustrative purposes only Technical specifications • Reprocessing prohibited • Sterilization validity: 3 years Models The full list of models and codes is available in the user manual. MANUAL DOUBT CATALOG VIDEO Related Products edit post Razek Scapel Blade edit post Razek Diamond Drills edit post Mini Microdebridement Cannula – ENTP edit post Cannula for Aggressive Bone Lesion
TFCKit

Home Products TFCKit ANVISA No.: 80356130159Images for illustrative purposes only TFCKit TFCKit has the function of providing the necessary devices to help suture intra-articular lesions in arthroscopic surgeries in general. ANVISA No.: 80356130159Images for illustrative purposes only Technical specifications • Single-use product • Sterilization validity: 3 years Items TFCKit (Código 760140000) 1. Loop Hook2. Long Curved Hook3. Short Curved Hook4. Straight Hook5. Graduated Probe MANUAL VIDEO DOUBT CATALOG Related Products edit post Razek Surgical Cutters edit post Pain Cannula STP Plus Kit edit post Razek Disposable Tourniquet edit post RZ Pump Irrigation Device
Foot RZ Traction Mesh

Home Products ANVISA No.: 80356130146Images for illustrative purposes only The Foot RZ Traction Mesh is a device designed to distribute the weight of the foot and traction it, enabling the surgeon to position the patient according to the requirements of foot and ankle surgical procedures. ANVISA No.: 80356130146Images for illustrative purposes only Technical specifications • Single-use product • Sterilization validity: 3 years Model • Traction Mesh Foot RZ – U(Code 742600100) MANUAL DOUBT CATALOG VIDEO Related Products edit post RZ Pump Irrigation Device edit post Razek Surgical Cutters edit post Razek Diamond Drills edit post Disposable Kit for Razek Traction Tower
Razek Blade STC

Home Products ANVISA No.: 80356130008Images for illustrative purposes only The Razek Blade STC has function is to cut fibrous tissue in percutaneous video arthroscopy procedures aimed at microsurgical treatment of compressive neuropathies (tumor, inflammatory, Morton’s neuroma, etc.), carpal tunnel syndrome, microsurgery of peripheral nerves (tarsal tunnel syndrome), fasciotomy or resection of plantar fascia (plantar fasciitis) and microneurolysis/neurolysis of compressive syndromes. ANVISA No.: 80356130008Images for illustrative purposes only Technical specifications • Single-use product • Sterilization validity: 3 years Composition Razek Blade STC(Code: 760010000)Composed of a cutting blade and a guide cannula. The cutting blade is located inside the guide cannula. MANUAL DOUBT CATALOG VIDEO Related Products edit post Cutters for Foot Surgery edit post Razek Pump Irrigation Device edit post Razek Diamond Drills edit post Mini Microdebridement Cannula

