FastFit Anchor Razek – Adjustable
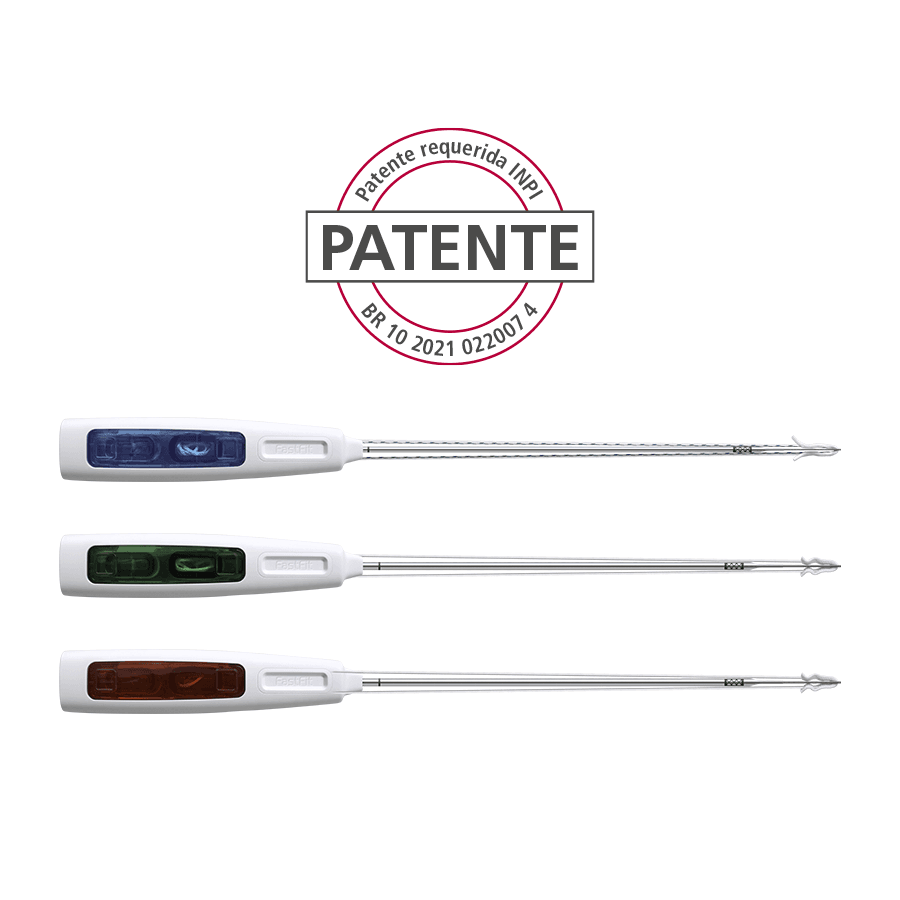
Home Products ANVISA No.: 80356130205Images for illustrative purposes only FastFit Anchor Razek – Adjustable The FastFit Anchor Razek flexible suture anchor family is indicated for use in arthroscopic or conventional orthopaedic surgical procedures on the upper and lower limbs, in which sutures are used to join soft tissues such as ligaments, tendons or joint capsules to the bone. Its design is based on the principles of minimal aggression and greater holding power. Reduced bone removal – the smaller diameter drill makes the hole created for the implant much smaller. As a result, there is greater preservation of the bone-tissue contact surface, as well as less aggression, considerably reducing post-surgical pain; Complications such as fractures, ostolysis and synovitis, which can occur with other materials, especially bioabsorbable materials, are minimized. The reduced diameter of the anchor reduces the need for larger bone perforations, which are common with metal, peek and bioabsorbable anchors, minimizing complications such as bone fragility and relesions. In addition, in cases where more anchors are needed, there will be less chance of complications and a greater possibility of their proper positioning; With the implant based entirely on sutures, the possibility of intra-articular free bodies that can occur in other types of implants is eliminated. ANVISA No.: 80356130205Images for illustrative purposes only Technical specifications • Single-use product • Sterilization validity: 3 years • Available in three diameters: 1.6 mm, 2.0 mm and 2.5 mm – loaded with very high-strength wires (UHMWPE * ForceFiber® Teleflex*); Models FASTFIT ANCHOR RAZEK – 1.6 mm Adjustable1.6 Adjustable (FAA 11-16S) – (Code 500120050)1.6 Adjustable (FAA 11-16M) – (Code: 500120055)1.6 Adjustable (FAA 11-16L) – (Code 500120057) FASTFIT ANCHOR RAZEK – 2.0 mm Adjustable2.0 Adjustable (FAA 12-20M) – (Code 500120040)2.0 Adjustable (FAA 12-20L) – (Code 500120045) FASTFIT ANCHOR RAZEK – 2.5 mm Adjustable2.5 Adjustable (FAA 12 25M) – (Code 500120100)2.5 Adjustable (FAA 12 25L) – (Code 500120105) MANUAL CATALOG VIDEO DOUBT Related Products edit post NitFix Razek edit post Cannulated Compression Screw MM Razek edit post Razek Snap Off Screw edit post FastFit Anchor Razek
Razek Endoscopes for Arthroscopy

Home Products ANVISA No.: 80356130121Images for illustrative purposes only Razek Endoscopes for Arthroscopy are medical devices designed to visualize openings and cavities during arthroscopic surgery. These instruments should be used in conjunction with Duet HD, Triplet HD equipment or any other surgical equipment that requires clear images for the surgical procedure. Razek Sterilizing Case is not part of the product and can be purchased separately. ANVISA No.: 80356130121Images for illustrative purposes only Models Endoscope Angled endoscope The full list of models is presented in the user manual. MANUAL DOUBT CATALOG VIDEO Related Products edit post Hip Arthroscopy Instruments edit post Razek Screw Instruments edit post Instruments for Implantable Materials – NitFix Razek edit post Razek Arthroscopy Instruments – Shoulder, Hip and Knee
FastFit Anchor Razek (Model with tape)

Home Products ANVISA No.: 80356130205Images for illustrative purposes only FastFit Anchor Razek (Model with tape) The FastFit Anchor Razek flexible suture anchor family is indicated for use in arthroscopic or conventional orthopaedic surgical procedures on the upper and lower limbs, in which sutures are used to join soft tissues such as ligaments, tendons or joint capsules to the bone. Its design is based on the principles of minimal aggression and greater holding power. ANVISA No.: 80356130205Images for illustrative purposes only More Details Tape models offer a larger contact surface, providing superior clinical results*. The tapes are extremely resistant, made from ultra-high molecular weight polyethylene. *Maia Dias, C., Gonçalves, S.B., Completo, A. et al. Why are tapes better than wires in knotless rotator cuff repairs? An evaluation of force, pressure and contact area in a tendon bone unit mechanical model. J EXP ORTOP 8, 9 (2021). https://doi.org/10.1186/s40634-020-00321-y Technical specifications Single-use product Sterilization validity: 3 years Ø 1.6mm1.6 (FTB 12-16M) – (Code 500120260)1.6 (FTA 12-16M) – (Code 500120265)1.6 (FTB 12-16L) – (Code 500120280)1.6 (FTA 12-16L) – (Code 500120285) Ø 2.5mm2.5 (FTP 22-25M) – (Code 500120270)2.5 (FTP 22-25L) – (Code 500120290) Model with needle2.5 (FTP 22-25MA) – (Code 500120275) Inserting device shank lengthM: 145 mm / L: 255 mmTape Width: 1.5 mm MANUAL CATALOG DOUBT VIDEO Related Products edit post FastFit Anchor Razek (models 1.5 and 1.5 Mini) edit post Fastfit Suture Razek edit post Feet RZ Rigid Fixation Plate System edit post FastFit Razek
FastFit Anchor Razek (models 1.5 and 1.5 Mini)
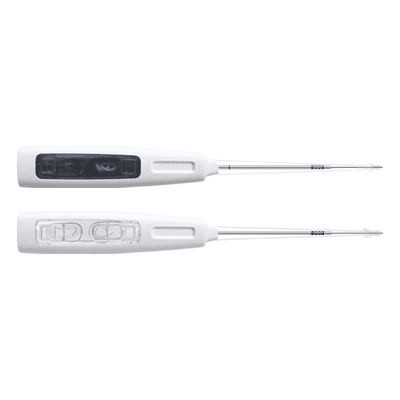
Home Products ANVISA No.: 80356130205Images for illustrative purposes only Indications for use: Temporomandibular Joint (TMJ), Foot and Ankle, Hand and Wrist FastFit Anchor Razek is a flexible suture anchor that guarantees maximum fixation with minimal bone aggression. The design is minimally invasive and uses highly resistant materials. This guarantees high stability and fewer complications in the post-operative period. The product’s instruments are purchased separately. ANVISA No.: 80356130205Images for illustrative purposes only Technical specifications • Single-use product • Sterilization validity: 3 years • Safe for use in an MRI environment Models • 1.5 Mini (FFM 10-15S) – (Code 500120115)• 1.5 (FFA 11-15S) – (Code 500120125) Adjustable models• 1.5 Mini Adjustable (FAM 10-15S) – (Code 500120110)• 1.5 Adjustable (FAA 10-15S) – (Code – 500120120) Models with needles• 1.5 Mini (FFM 10-15SA) – (Code 500120210)• 1.5 (FFA 11-15SA) – (Code 500120215) MANUAL CATALOG DOUBT VIDEO Related Products edit post PG Razek Cannulated Compression Screw edit post Fastfit Suture Razek edit post FastFit Razek edit post HalluFix PG Razek
Nano Guillotine

Home Products Nano Guillotine ANVISA No.: 80356139024Images for illustrative purposes only Nano Guillotine Nano Guillotine is a cannulated device for cutting, removing and suctioning tissues such as cartilage, fibrocartilage, synovial membranes and soft tissues in general, in different cavities and joints such as the shoulder, wrist, knee and ankle. The integrated storage chamber allows the removed tissue to be stored for later analysis by biopsy, quantification or visual analysis. Designed for use in space-saving surgeries that require tissue removal. ANVISA No.: 80356139024Images for illustrative purposes only Technical specifications • Single-use product • Sterilization validity: 2 years Models Nano Guillotine Kerr C – Size 80 mm x 3.5 mm (Code 921290000) Nano Guillotine Kerr L – Size 150 mm x 3.5 mm (Code 921290100) Nano Guillotine Wedge C – Size 80 mm x 3.5 mm (Code 921290200) Nano Guillotine Wedge L – Size 150 mm x 3.5 mm (Code 921290300) Nano Guillotine Hook C – Size 80 mm x 3.5 mm (Code 921290400) Nano Guillotine Hook L – Size 150 mm x 3.5 mm (Code 921290500) MANUAL CATALOG DOUBT VIDEO Related Products edit post Suture Needle edit post Puncture Cannula edit post Razek Surgical Cutters edit post Suture Fix Access Cannula
FastFit Knotless Razek

Home Products FastFit Knotless Razek ANVISA No.: 80356130211Images for illustrative purposes only FastFit Knotless Razek The FastFit Knotless Razek family composed of anchor-type implants indicated for the fixation of soft tissue to bone, acting to support tensile loads through compression between the anchor and bone tissue when implanted. It is indicated for use in arthroscopic or conventional orthopaedic surgical procedures on the upper and lower limbs, in which the anchor, using sutures, is used to join soft tissues such as ligaments, tendons or joint capsules to the bone. The implants in this family are composed of an anchor – comprising the Anchor Body and Anchor Eye – made from Polyetheretherketone. (PEEK). The FastFit Knotless Razek family anchors are suspension fixation elements whose combination of anchor and suture allows soft tissue to be reinserted into the bone, enabling stabilization, repair and reconstruction, acting as a fixation pole or distributing tensile loads. To do this, the anchor is impacted into the bone tissue together with the sutures. The sutures attached to the anchor, in turn, are sutured to the soft tissue so that it can be fixed and regenerated. ANVISA No.: 80356130211Images for illustrative purposes only Technical specifications • Single-use product • Sterilization validity: 3 years Models D2.9 – Sizes Ø 3.2 x 15.99 mm (Code 500120300) D3.5 – Sizes Ø 3.5 x 20.3 mm (Code 500120305) D4.5 – Sizes Ø 4.6 x 23.8 mm (Code 500120310) MANUAL CATALOGO DOUBT VIDEO Related Products edit post Upper Treat RZ Rigid Fixation Plate System edit post Fastfit Suture Razek edit post PG Razek Cannulated Compression Screw edit post FastFit Anchor Razek – Adjustable
FastFit Razek

Home Products FastFit Razek ANVISA No.: 80356130207Images for illustrative purposes only FastFit Razek The FastFit Razek family implants are composed of a flexible suture anchor and a cortical plate, interconnected by an adjustable suture, and are indicated for reapproximation and stabilization between bone structures in different arthroscopic or conventional orthopedic surgical procedures. The unique combination of the flexible suture anchor and the cortical plate results in a suspension fixation element that acts as a stabilization pole or distributes tensile loads. The adjustable suture, on the other hand, has an adjustable knot that makes it possible to quickly adjust the tension of the set and the distance between the bone structures. Compared to traditional button-button fastening systems, the FastFit Razek has the advantage of preserving the 4th cortical bone, resulting in a fixation with less removal of biological material and fewer prominences. ANVISA No.: 80356130207Images for illustrative purposes only Technical specifications Single-use product Sterilization validity: 3 years Anchor characteristics Available in 2.5 mm diameter – loaded with very high-strength wire (UHMWPE); Reduced bone removal – the smaller diameter drill makes the hole created for the implant much smaller. As a result, there is greater preservation of the bone-tissue contact surface, as well as less aggression, considerably reducing post-surgical pain; Complications such as fractures, ostolysis and synovitis, which can occur with other materials, especially bioabsorbable ones, are minimized; The reduced diameter of the anchor reduces the need for larger bone perforations, which are common with metal, peek and bioabsorbable anchors, minimizing complications such as bone fragility and relesions. In addition, in cases where more anchors are needed, there will be less chance of complications and a greater possibility of their proper positioning. Characteristics of Cortical Plates • Cortical plates available in 8 models for different applications; • The STD cortical plate has a geometry that allows its passage through bone tunnels with the aid of a passage suture. It is important to emphasize that the passage suture supplied with the STD cortical plate has the sole function of assisting the passage of the plate through the bone tunnel and should be removed after this step, not remaining implanted. • The ACB cortical plate has a geometry that allows perfect accommodation in the most diverse bone structures. It has a large surface area, providing excellent stress distribution on the cortical surface. • The DCB cortical plate has a geometry similar to the ACB cortical plate, but has lateral slots that allow temporary disassembly of the assembly. This feature allows the passage of the adjustable suture through reduced bone tunnels. • The ACP cortical plate has a geometry that allows it to fit into the holes of the FastFit Razek Contact Plates, as well as the compression holes of the UpperTreat RZ Rigid Fixation System plates (ANVISA nº 80356130215). It offers the surgeon the option of a combined repair. • The DCP cortical plate has a geometry similar to the ACP cortical plate, but with lateral slots that allow temporary disassembly of the assembly, enabling the passage of adjustable sutures through reduced bone tunnels. • The ASP cortical plate has a geometry that allows it to fit into the compression holes of the Feet RZ Rigid Fixation Plate System plates (ANVISA nº 80356130162), offering the surgeon the option of a combined repair. • The DSP cortical plate has a geometry similar to the ASP cortical plate, but features lateral slots that allow for temporary disassembly of the assembly, enabling the passage of adjustable sutures through reduced bone tunnels. • The DMB cortical plate has a reduced geometry, allowing for perfect accommodation in small bone structures, with excellent stress distribution on the cortical surface. In addition, it has lateral slots that allow for temporary disassembly of the assembly, enabling the passage of adjustable sutures through reduced bone tunnels. • Cortical plates and contact plates manufactured from ASTM F136 titanium alloy. Models FastFit Razek – 2.5 Adjustable STD (FST 15-25M)(Code 500120015) FastFit Razek – 2.5 Adjustable ACP (FAP 15-25M)(Code 500120020) FastFit Razek – 2.5 Adjustable ACP (FPC 15-32M)(Code 500120021) FastFit Razek – 2.5 Adjustable ACP (FPC 15-36M)(Code 500120022) FastFit Razek – 2.5 Adjustable ACP (FPC 15-40M)(Code 500120023) FastFit Razek – 2.5 Adjustable ACP (FPC 15-25M)(Code 500120030) MANUAL CATALOG VIDEO DOUBT Related Products edit post FastFit Knotless Razek edit post EuroScrew edit post Cannulated Compression Screw MM Razek edit post NitFix Razek
Hip Arthroscopy Instruments

Home Products n.º ANVISA: 80356130177, 80356130034 e 80356130121Imagens meramente ilustrativas Its function is to provide the surgeon with the necessary instruments to carry out procedures in arthroscopic hip surgery. ANVISA No.: 80356130177, 80356130034 and 80356130121Images for illustrative purposes only Items Access Trocar (Code 930360330) Guide Cannula 4 mm (Code 930360250) Guide Cannula 5.5 mm (Code 930360240) Introducer Guide (Code 930360260) Cannula Trocar 90 (Code 930360340) Cannula Trocar 110 (Code 930360350) Angled Guide (Code 930360300) Access Guides– Access Guide 4.5 (Code 930360290)– Access Guide 5.5 (Code 930360280)– Access Guide 6.5 (Code 930360270) Blunt Access Cannulas– Blunt Access Cannula 3.5 (Code 930360290)– Blunt Access Cannula 4.5 (Code 930360190)– Blunt Access Cannula 5.5 (Code 930360180) Oblique Access Cannulas– Oblique Access Cannula 3.5 (Code 930360170)– Oblique Access Cannula 4.5 (Code 930360160)– Oblique Access Cannula 5.5 (Code 930360150) Perforating Access Cannulas– Perforating Access Cannula 3.5 (Code 930360230)– Perforating Access Cannula 4.5 (Code 930360220)– Perforating Access Cannula 5.5 (Code 930360210) Probe Short Q (Code 930360310) Probe Long Q (Code 930360320) Long Knot Pusher (Code 930360410) Short Knot Pusher (Code 930360400) Suture Hook (Code 930360390) Ice Pick Straight (Code 930360360) Ice Pick 30° (Code 930360370) Ice Pick 45° (Code 930360380) Banana Knife 1 Cut (Code 930360420) Banana Knife 2 Cuts (Code 930360430) Interchangeable RZ trocar 70º x 175mm x 4.00mm – Obturator cannulated tip(Code 700421200) Endoscope 175 mm / 30° / 4.0 mm (Code 742401400) Endoscope 175 mm / 70° / 4.0 mm (Code 742401500) Pinches– Bird beak, straight tip (R0052) – 220mm (Code 741830002)– Bird beak, up 30° tip (R0054) – 220mm (Code 741850002)– Bird beak, straight tip, curved right 30° (R0058) – 220m (Code 741890002)– Grasper, straight tip, 3,4 mm bite width, loose body (R022) – 220mm (Code 740360002)– Punch, straight tip, 3,6 mm bite width (R003) – 220mm (Code 740170002)– Bird beak, straight tip, curved left 30° (R0056) – 220mm (Code 741870002)– Suture cutter, straight tip (R0060) – 220mm (Code 741910002) Razek Sterilizing Case – CX 015 (Code 700431120) MANUAL DOUBT CATALOG VIDEO Related Products edit post Razek Irrigation Cannula edit post FitPass (R084) edit post Razek Screw Instruments edit post Trocar RZ
Meniscus Suturing Instruments

Home Products ANVISA No.: 80356130177Images for illustrative purposes only The Meniscus Suture Instruments are designed to provide the surgeon with instruments to help suture the meniscus. ANVISA No.: 80356130177Images for illustrative purposes only Items • Single Cannula (Code 930360030) • Straight Double Cannula (Code 930360040) • Double Cannula Up (Code 930360050) • Double Cannula Down (Code 930360060) • Double Cannula Right (Code 930360070) • Double Cannula Left (Code 930360080) • Pusher (Code 930360090) • Razek Sterilizing Case – CXPL 007 (Code 700439400) MANUAL DOUBT CATALOG VIDEO Related Products edit post FitPass (R084) edit post Razek Endoscopes for Arthroscopy edit post Tunneling Tools edit post Trocar RZ
Razek Screw Instruments

Home Products ANVISA No.: 80356130055Images for illustrative purposes only The purpose of the Razek Screw Instrument family is to provide the surgeon with the necessary instruments for inserting and fixing interference screws in anterior cruciate ligament and posterior cruciate ligament reconstruction surgeries. ANVISA No.: 80356130055Images for illustrative purposes only Models • Hexagon Socket Key 30 (Code 742030000)Wrench with 30 mm hexagonal tip, used for inserting 30 mm long interference screws. The full list of models is presented in the user manual. MANUAL DOUBT CATALOG VIDEO Related Products edit post Hip Arthroscopy Instruments edit post Tunneling Tools edit post Trocar RZ edit post Razek Arthroscopy Instruments – Shoulder, Hip and Knee

